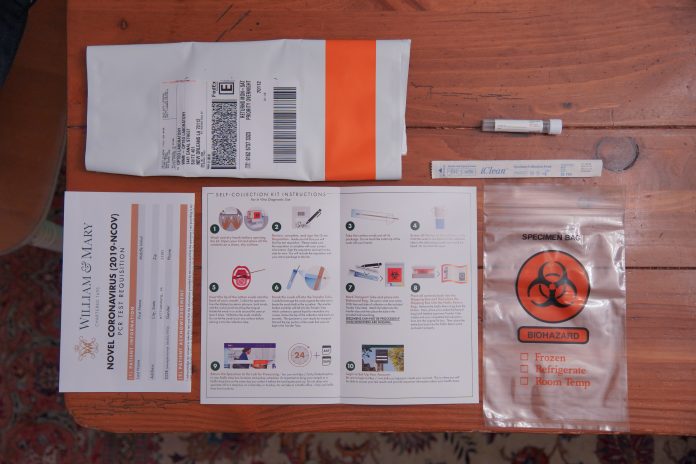
Before new students moved into campus Aug. 12 and in advance of returning students’ anticipated arrival in Williamsburg after Labor Day, the College of William and Mary has partnered with VCU Health to administer COVID-19 testing to all students returning to campus for the fall semester. According to the College’s health protocols, all students must test negative before returning to campus or risk being turned away upon arrival.
VCU Health contracted with Kallaco Health & Technology to administer testing. Recently, Kallaco has come under fire from some students, who have claimed their tests are not being processed quickly enough. Students and faculty members at other Virginia universities have also intensified questioning of Kallaco’s credentials and the tests’ accuracy.
College biology professor and virologist Kurt Williamson warned that proper tests are extremely important. Williamson echoed the concerns raised by faculty of Virginia Commonwealth University and George Mason University that the tests students received are not FDA authorized for home collection of samples.
“Yes, students should be concerned,” Williamson said in an email. “Students should be asking questions right now.”
“Yes, students should be concerned, Students should be asking questions right now.”
In response to these allegations, Kallaco spokeswoman Amy Cheronis emphasized the company’s reputability.
“Kallaco was created by U.S. healthcare executives who saw firsthand what the lack of testing could do to highly-impacted areas while they were working in New Orleans when COVID-19 struck,” Cheronis said in an email. “They were able to deliver testing to one of the first, and hardest hit, areas of the city. They believed this lack of available testing was going to quickly and dramatically affect businesses and universities across the country. Members of the Kallaco team have worked in healthcare for decades, supporting universities, hospitals and employers in many different capacities.”
Testing is a major component of the College’s plan for returning to campus. Many students and faculty are still uneasy about the new company, and question whether test kits are being processed quickly and accurately enough to be effective preventative measures against the spread of COVID-19 on campus.
Late Tests and Move-In Troubles
Many students at the College did not enjoy smooth experiences with Kallaco’s COVID-19 testing.
“My experience with testing was stressful and slightly complicated,” Sarah Larimer ’23 said in an email. “I had sent in my test the day I received it because I was approved to move in early. Based on the original time frame given for results (within 36 hours), I should have had plenty of time between getting my results and then leaving for VA. However, it took around 80 hours for me to get my results. This was hugely problematic for me.”
Larimer, who lives in Chicago, had to begin her 14-hour drive to Williamsburg without having received her test results. Though Larimer received her negative result before she moved in, others were not as lucky.
Soon after Larimer arrived on campus, Residence Life informed students via email that they could use negative results from third-party providers, a shift from the College’s previous mandate that all results had to come from Kallaco.
“I think that all the testing protocols were done with the best interest of the students in mind, but with everything changing so quickly in the course of a week, adaptive measures didn’t change at the same pace,” Larimer said. “I was super lucky, my results came in negative and I was able to keep my move-in time. But I had to go against the school’s recommendation of not leaving home until I had a negative test. And I have no idea what I would have done if I didn’t test negative. I think that the changes they recently made are a good step, but didn’t come in time for a lot of students.”
Maggie Herndon ’22 had been expecting to move in during Labor Day weekend, like most upperclassmen, but was informed by the College Aug. 7 that she could move in early.
Many students attempted to communicate their problems with testing to the College. Larimer initially encountered difficulties when interacting with administrators.
“I had mixed interactions when it came to contacting the school. So when I first contacted the school I was told to be patient and that I could always change my move in date. This was extremely frustrating.”
“I had mixed interactions when it came to contacting the school,” Larimer said. “So when I first contacted the school I was told to be patient and that I could always change my move in date. This was extremely frustrating.”
After emailing a number of administrators, Larimer was contacted by Senior Associate Dean of Students Mark Sikes.
“I was then contacted by somebody from the Deans’ office who told me that I could expect my results that night and basically walked me through my options,” Larimer said. “This phone call from Dean Sikes was lifesaving. I cannot articulate to you how helpful he was. For the first time in the process I felt like I was actually talking to a real person who understood the situation I was in.”
Though Larimer’s conversation with Sikes alleviated her frustration, she also noted a lack of any contact from Kallaco, whom she contacted but did not hear back from.
Herndon also contacted the College about her troubles with testing and found the College’s move-in hotline to be helpful. Despite being able to ultimately move in, Herndon questioned the partnership with Kallaco.
“I think the University could have been more up front about the fact that there are concerns about the validity of Kallaco’s tests, and that not much is known about the company,” Herndon said. “I think they also could have been more clear that test results may not actually be available until well over 72 hours after delivery. I think they made a good decision by allowing students to take other tests, but I think they could have been more proactive in making sure students know how to access other tests.”
According to Chief Operating Officer Amy Sebring, multiple factors played into delayed Kallaco results, including faults made by students while taking and sending the tests and administrative delays on the College’s end.
“The turnaround time for test results for our first students arriving on campus was tight and we did experience testing delays,” Sebring said in an email. “Some of those delays were due to delays in W&M shipping students the test kits, students completing the required registration, incomplete samples being presented for testing and delays in the lab response time, consistent with what we have seen nationally due to high demand.”
Sebring mentioned that Kallaco worked double-time to meet the College’s demand, pointing to the call center and backup testing options as steps taken to ensure students were able to meet their move-in deadlines. Sebring does not anticipate the same issues as returning students prepare to move in during early September and said that Kallaco’s turnaround time now consistently lies within a 72-hour window.
The College is not alone in experiencing concerns regarding Kallaco testing. A widely circulated Aug. 20 letter from faculty at GMU and VCU urged the Virginia Department of Health to investigate Kallaco’s credentials as a company.
“It seems odd to us that this young IT company, which has been awarded sole source state contracts with a potential value of over $4 million, has been entrusted with the critical COVID-19 testing for up to 28,500 students at our universities.”
“It seems odd to us that this young IT company, which has been awarded sole source state contracts with a potential value of over $4 million, has been entrusted with the critical COVID-19 testing for up to 28,500 students at our universities,” the letter said.
In an email to GMU faculty that was obtained by The Flat Hat, members of the faculty senate said VDH declined to investigate, as the department was not involved in the contract’s negotiation.
In a different email obtained by The Flat Hat, President of the Faculty Senate of Virginia and VCU Professor Carmen Rodriguez said that she asked the president of the faculty senate to sign the letter, but was in a hurry to send the letter to VDH. No members of the College’s faculty senate ultimately signed the letter.
According to the company’s LinkedIn page, Kallaco was founded in April 2020 by John Spivey. The for-profit, New Orleans-based company describes itself as a comprehensive software solution for testing and monitoring of employee and student health. It identifies its clients as employers, universities and hospital systems.
Spivey holds a PhD in American studies from the University of Kansas, according to his website. His past business ventures focus mainly on technology and healthcare services. Spivey is concurrently the CEO of both Kallaco and his private consulting firm, Spivey & Company.
While Spivey has dabbled in the healthcare industry, he is primarily an executive with no scientific background. According to Kallaco’s website, San San Ng, who holds a PhD in human genetics and has a background in clinical testing, is the company’s Chief Scientific Officer. Other members of the executive team include various advisors and consultants from partner companies.
As a new company with little public information, students have expressed skepticism about the College’s partnership with Kallaco.
“I have some serious concerns about the Kallaco tests, given that there is not much information about the company on their website,” Herndon said. “I am not a scientist and am certainly not capable of determining whether or not the tests are reliable, but I think the school should have screened the company better before hiring them.”
The faculty from VCU and GMU also expressed concerns over the tests themselves, claiming that some tests were not given FDA Emergency Use Authorization. Williamson echoed these concerns.
“I have heard from at least two students who have told me that the tube for swab storage was labeled “NOT FOR DIAGNOSTIC PURPOSES” which matches up with the experiences described at the other schools,” Williamson said. “Yes, we should be concerned.”

Cheronis told The Flat Hat that the label, “not for diagnostic purposes,” refers to the tube in the kit, which is not a diagnostic device.
Kallaco responded to inquiries by releasing a statement on its website, which included answers to frequently asked questions. According to the statement, testing for Virginia universities is conducted at Opteo Laboratories, a Clinical Laboratory Improvement Amendments certified facility in New Orleans. A Center for Disease Control and Prevention database search confirmed that the lab is indeed CLIA certified.
In the statement, Kallaco firmly emphasized that all its testing is FDA authorized but did not specify which test was sent to students at the College or other universities.
“The testing provided by Kallaco has all of the licenses required in the U.S.,” the Kallaco statement said. “As an extra step, the testing was submitted to the FDA and the Kallaco labs have the authority to run the test under FDA EUA guidelines.”
The faculty letter claimed students received the Thermo Fisher TaqPath COVID-19 Combo Kit IFU but did not specify how this information was obtained. The Flat Hat attempted to contact Opteo Laboratories multiple times but received no response.
The FDA first granted the TaqPath test an EUA March 13. It underwent several modifications between March and July. Cheronis said students were sent the modified version. Aug. 17, the FDA released a warning saying that the TaqPath test may lead to inaccurate results, specifically with issuing false positives. The warning cites “inadequate vortexing and centrifugation of RT-PCR reaction plates” as the reason for the inaccuracies. The warning does not mention home specimen collection as a cause for false positive results.
While the TaqPath test has been granted an EUA by the FDA, it is not authorized for home collection of samples according to an FDA database. Thermo Fisher, the company that developed the test, confirmed in a phone call that the TaqPath COVID-19 Combo Kit cannot be used with home collected samples.
Aug. 23, College spokesperson Suzanne Clavet provided The Flat Hat with a statement claiming Kallaco has assured them that the test kits were FDA approved for at home collection.
“We have been assured by Kallaco that their labs are CLIA approved and the tests kits provided to all university partners have been approved by the FDA for home collection,” Clavet said in an email. “These are unprecedented times and we understand the questions. At-home collection kits are more variable and for that reason we have repeated tests where results were inconclusive. We remain in constant contact with the company CEO. The company is committed to providing concrete answers to any concern raised.”
Cheronis later confirmed that the test sent to students was indeed the Thermo Fisher TaqPath COVID-19 kit for its COVID-19 assay, but it was modified by Opteo Laboratories. The result is a Laboratory Developed Test, and in recent months, criticism regarding LDTs has increased, especially after the Trump administration rolled back the FDA’s ability to regulate LDTs last week.
“Please note that LDTs should not be used for clinical diagnoses without FDA’s approval, clearance, or authorization during an emergency declaration for that disease,” the FDA website said.
The test that Cheronis is referring to is Opteo’s version of an LDT. It remains on a list of LDTs that have been submitted to the FDA but are awaiting EUA. As of this writing, the Opteo LDT has not been reviewed by the FDA and is listed as “not FDA authorized.”
“The collection kits used by Opteo Laboratory have been internally validated as a Laboratory Developed Test (LDT) and submitted to the FDA under the EAU guidelines,” Cheronis said. “Opteo’s EUA submission on April 15, 2020 allowed for the specimen to be self-collected via an oropharyngeal swab.”
Though the Opteo test is derived from an FDA EUA test and has been developed to allow for self-collection, the FDA has not confirmed its efficacy. Essentially, the test remains on a waiting list for EUA.
Some students and faculty worry that home specimen collection may become an issue for students completing their tests before returning to campus.
“I’m not at all sure that I did it correctly,” Gorski said, referring to her Kallaco COVID-19 test. “I mean they provided instructions but I certainly know I did a very unprofessional job. I think I know a lot of other people who feel the same way, which is obviously a problem for the accuracy of the test.”
Williamson validated this concern and said that an improper collection of a sample could result in a false negative. He said at-home testing can be done effectively, but only if the kits are designed for that purpose.
“The short answer is that if you don’t swab the right part of your respiratory tract in the right way, you could get a false negative,” Williamson said. “Some tests assume that a nasopharyngeal swab has been used — this is the “tickle your brain” swab that goes way back. Other tests assume a pharyngeal or throat swab – this is the same as a strep test, for anyone who has had that unpleasant experience. Neither of these swabs can be reliably self-administered, and attempts to do so may end up contaminating the swab and invalidating the test. For example, if you were attempting to swab your own throat, if you accidentally hit your tongue, you will pick up enzymes that will destroy any viral particles you might have also picked up.”
Determining the Best Path Forward
Some universities requiring pre-arrival testing have chosen to partner with more established companies. Elon University and the University of Notre Dame, among other colleges, have partnered with LabCorp, which was founded in 1978.
In contrast to LabCorp’s history in the clinical testing field, the partnership with VCU Health appears to be Kallaco’s first and largest. Among the three universities partnered with VCU Health, Kallaco claims to have administered over 10,000 tests. Cheronis said the company has administered 10,000 more outside of the universities.
“Together with VCU Health System, our health services partner, we selected Kallaco to provide those testing services using the state’s procurement bid process,” Sebring said. “While Kallaco is formally newly established, the founders have a long history in software development and the healthcare space. One of the many reasons William & Mary selected Kallaco as a partner is their relationship with a robust network of national labs.”
Mason School of Business professor Graham Henshaw said that a startup like Kallaco may be a better partner for universities than more established companies like LabCorp.
“Being an early adopter often has many benefits that aren’t possible when dealing with legacy companies,” Henshaw said in an email. “Having the direct attention of the CEO of this company will enable much faster responses to our specific needs than if W&M was just one additional customer among hundreds of others.”
“Having the direct attention of the CEO of this company will enable much faster responses to our specific needs than if W&M was just one additional customer among hundreds of others.”
Henshaw emphasized the importance of Kallaco’s team in the College’s decision to contract them.
“While Kallaco appears to be a “young” company, their leadership has decades of experience in the health technology sector,” Henshaw said. “From a startup perspective, you derive most of your execution confidence from the team. In this case, the team also includes VCU Health which is an incredible partner to have on this effort because they bring the full support of a world-class health system to bear.”
Henshaw explained that universities looking for testing and healthcare partnerships in the era of COVID-19 might logically be pushed towards startups.
“For a partnership like this, responsiveness is key,” Henshaw said. “We’ve experienced that agility is required to navigate this pandemic. I would look for a partner that could be as agile as required by the rapidly changing situation we face, which would point me in the direction of a startup.”
Nevertheless, students argue that the College faces pressing economic problems and must address the needs of its students, staff and faculty first and foremost. Compounded with concerns about the validity of tests, some still question whether the partnership with Kallaco achieves the goal of a safe reopening.
“I am concerned that William and Mary has given $194,392 (according to the Richmond Times Dispatch) to Kallaco so far in spite of the overall lack of knowledge about the company, particularly in this time when some faculty and staff have already been laid off or furloughed, and many students are struggling to pay tuition due to the economic consequences of this pandemic,” Herndon said.
Other students say that testing frustrations exacerbate an already difficult situation. For many who yearn for a problem-free return to campus, late test results are just another complication.
“There were so many hurdles for me to come back to WM this past week,” Larimer said. “I had to fight to get my move-in exception approved. And to top that off I was supposed to live in OTP so I also obviously needed to change those plans too. And at the end of all of that I had hoped that my COVID test was going to be quick and easy. I was just so annoyed how the only thing that was going to stop me from going back wasn’t even my fault.”