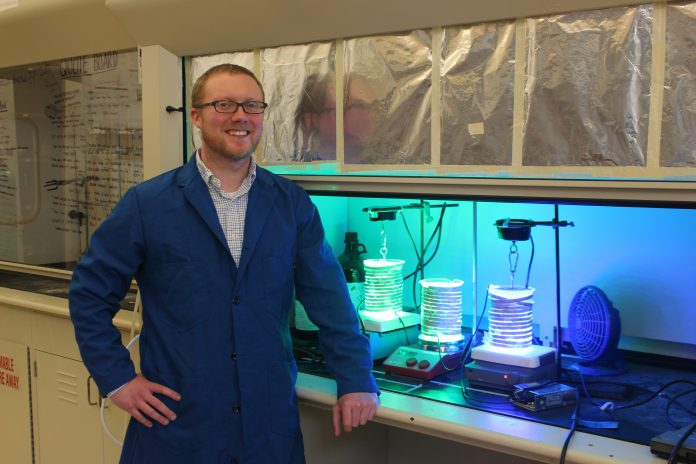
Professor William McNamara is filtering water from Lake Matoaka through a T-shirt to produce renewable energy in a matter of minutes. It’s a mission that is eight years in the making for his research lab and is powered by one of the earth’s biggest energy hotspots: the sun.
McNamara, an associate professor in the chemistry department, was inspired by plants’ natural energy-making processes to find a way to harvest solar energy.
“The thing that’s good about solar energy is that it’s the most abundant renewable energy source,” McNamara said. “More energy strikes the earth in the form of sunlight in an hour and a half than we use in a year.”
McNamara explained that many modern applications of solar energy involve storing it in batteries that are expensive and not energy efficient. Rather than using batteries, he chose to pursue storing the products of solar energy in a fuel that is produced by splitting water into its individual parts of hydrogen and oxygen.
“If we split water to give ourselves oxygen gas and hydrogen gas, you can burn hydrogen directly to get energy and water,” McNamara said. “If you can take some dirty water from a pond or a river, dump it in this reactor and split the water — now, you’ve got clean water from that reaction and energy. It’s kind of solving two problems at once.”
“If you can take some dirty water from a pond or a river, dump it in this reactor and split the water – now you’ve got clean water from that reaction and energy. it’s kind of solving two problems at once.”
The production of clean water as part of the reaction to create hydrogen gas is a component of the system that McNamara has not studied specifically, but it could be helpful for developing countries.
“If we can take water or dirty pondwater, something that’s abundant to people, turn it into energy, and then also be able to give them clean drinking water, that is such a big win,” McNamara said. “So that’s a nice side bonus of our energy emission and powering the planet: maybe we can do this in a way that would give us clean water, too.”
McNamara and his lab developed a working system that takes in solar energy, uses it to split water into hydrogen and oxygen and stores the resulting energy as hydrogen gas. Hydrogen energy fuel can be detected from the working model in as early as 10 minutes, and the energy lasts for up to 10 days once produced. McNamara tested the system using water from Lake Matoaka with successful results.
The energy-creation process begins when light strikes a dye on the system called a chromophore that is able to absorb that light. The chromophore then hands over a charged particle called an electron to jumpstart a miniature machine called an enzyme that helps split the water into hydrogen and oxygen.

Enzymes are biological boosters that are used to speed up chemical reactions for living things. The particular enzyme chosen by McNamara, iron hydrogenase, uses iron to accomplish its mission.
“Your blood, right now, is carrying oxygen around your body using iron,” McNamara said. “Our idea here was, why don’t we use an earth-abundant metal that is all around us, because then we are going to be able to do it on a wider scale and drive down costs?”
The enzyme is held steady on a solid support provided by titanium dioxide — a material also found in white paint, sunscreen and Doritos. This support system means that the enzyme is held still and stable for the duration of the water-splitting reaction and can be recycled for future reactions, as opposed to a one-and-done model.
“Once it’s done, you can take it out of solution and you can put it in another solution and use it again,” McNamara said. “It’s really wonderful.”
McNamara and his lab paid attention to sustainability during the process of developing the system, meaning that its individual parts are inexpensive and abundant in the environment. The energy production system might be useful in developing countries that do not have the ability to widely distribute costly energy-production devices. The system’s dependence on solar energy, a renewable source of power, is friendlier to the environment and could reduce human dependency on nonrenewable energy sources.
“The amount of energy we’re using as a globe is rapidly increasing as nations are getting more developed,” McNamara said. “Our current energy infrastructure is not sustainable for us for an infinite amount of time.”
“OUR CURRENT ENERGY INFRASTRUCTURE IS NOT SUSTAINABLE FOR US FOR AN INFINITE AMOUNT OF TIME.”
Members of McNamara’s lab will be presenting research on the project at the American Chemical Society’s annual meeting in Philadelphia, Pennsylvania in late March. McNamara is excited about the future of the project as well as the thrill of discovery during the research process.
“That feeling you get when you’re the first person to observe something — there’s nothing better than that,” McNamara said. “It’s like, no one else has ever made this. No one else has even seen this. It’s crazy; that’s crazy.”

[…] Click here to view original web page at Charging Up : William McNamara harnesses solar power for sus… […]